What future for AI in Europe? 🤖 | June 2022.
What future for AI in Europe? 🤖 | June 2022.
Jun 30, 2022 • 5 min read • Download article

.
As a company that has developed a solution based on Artificial Intelligence (AI) and neuroscience ans as manufacturer of Medical Devices (MD), myBrain Technologies has to stay up to date with regulations and standards in these fields.
For this, we can rely on our quality team! 🙏🏻
This month, we are proud to present the white paper of our Quality Director on the future of AI regulation in Europe 📗
We also talk about the difficulties and solutions related to the application of the Medical Device Regulation (MDR) 📃
Summary |
What future for AI in Europe? 📗 |
myBrain Tech’s standards 🏅 |
Eye on Medical Device Regulation 🎙 |
What future for AI in Europe?
#DownloadWhitePaper 📗


With the increasing evolution of computing, robotics, and new technologies, AI is now a major part of our daily life.
On April 21, 2021, the European Commission published a proposed regulation on AI, the Artificial Intelligence Act, which aims to establish safe and harmonized rules for this innovative field.
In her White Paper, Sophie Colliot, Quality Director at myBrain Technologies, reviews the main contributions, impacts and normative perspectives of the proposed regulation. She also compares the implementation of other regulations closely related to AI and specifies the various opinions of the CNIL and its counterparts on the subject, thus highlighting the possible fields of evolution.
Download the White Paper here >>
myBrain Tech’s standards for MDR
#Certification 🏅
At myBrain Technologies, we strive to ensure that our Quality Management System guarantees customer satisfaction through consistent and standardized processes together with centralized and organized quality control policies.
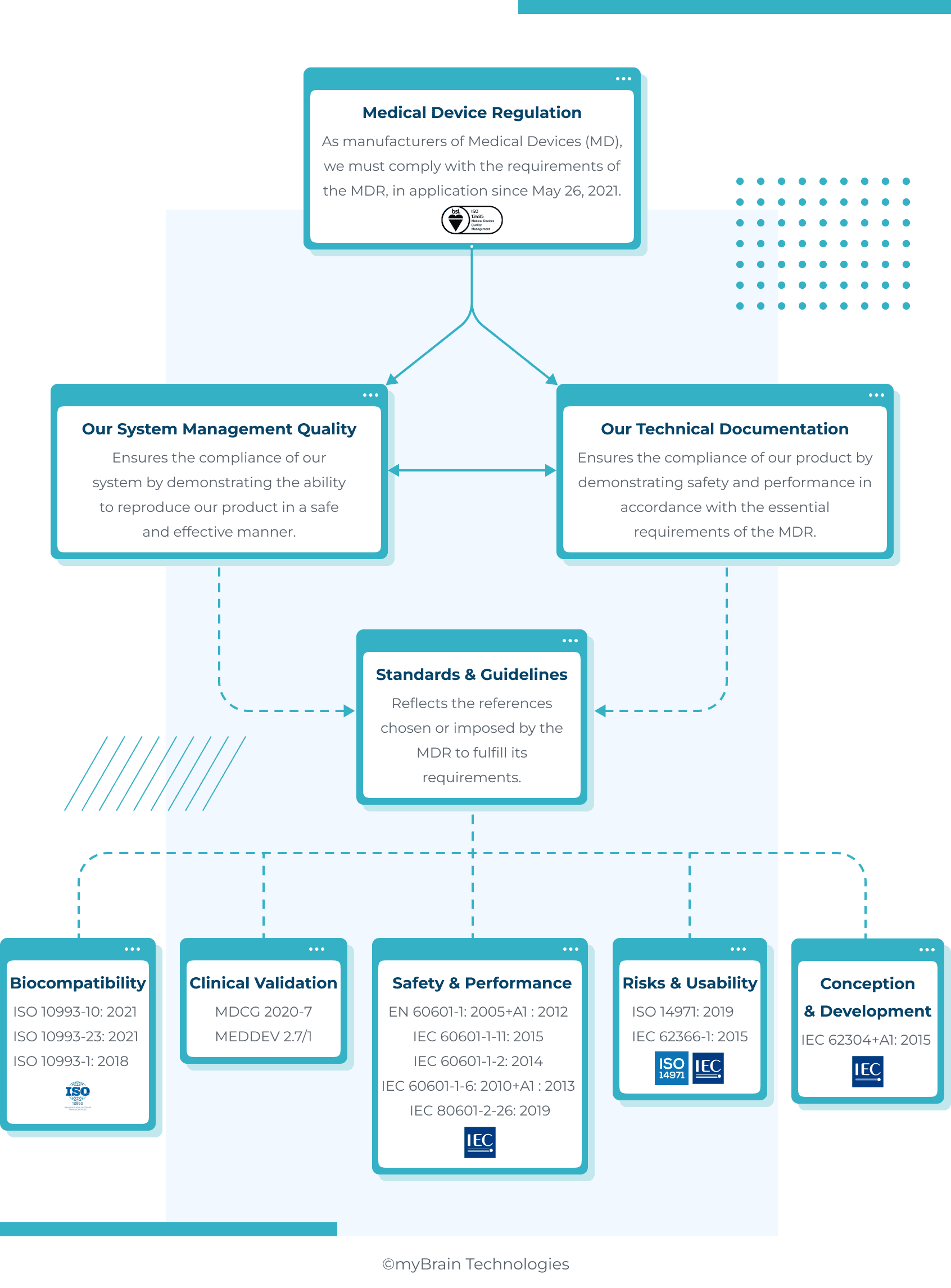
Focus on Medical Device Regulation
#Interview 🎙
Nowadays, if there is one sector in which AI systems are crating a major upheaval, it is the healthcare and Medical Devices sector.
With the aim of reinforcing health safety and harmonizing the application of the rules within EU, an in-depth revision of the regulations led to a new specific Medical Device Regulation (MDR 2017/745) – in application since May 26, 2021.
Bringing MDs to market, while complying with new regulatory requirements, poses challenges that legitimately worry manufacturers. However, there are strategies that can help them acquire the necessary certification to bring their products to market.
To understand these difficulties and find solutions, we interviewed Sophie Colliot, Quality Director at myBrain Technologies.
Discover the interview here >>

© 2022 myBrain Technologies, All rights reserved.
50 Avenue Claude Vellefaux
75010 Paris
Related Articles
Get in touch
We are glad you are interested in reaching out to us. Whether you have a question about our products or want to give us feedback, we are here to help.