The path to ISO 13485.
Implementing a quality approach within a start-up poses particular difficulties linked to the context of innovation, agility and rapid growth. However, such an approach is essential to guarantee the sustainability of its activities and to enable it to become a leader in its market.
Report.
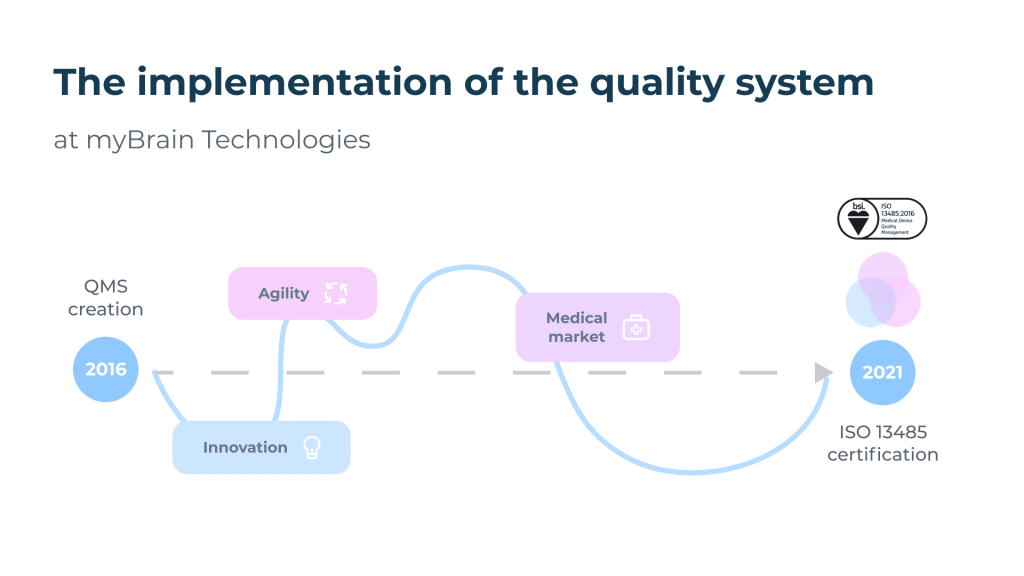
On June 2, 2021, myBrain Technologies obtains the ISO 13485 certificate (Medical devices – Quality management systems – Requirements for regulatory purposes). The entire team is very proud of this. The publication of the ISO 13485 certificate is synonymous with easier access to the market of safe and efficient medical devices, but it also means for us the concretization of 5 years of beautiful, rich and intense experiences from an organizational and human perspective.
Launch.
The quality process was initiated in 2016 at myBrain Technologies, only two years after its creation, and following the completion of my second university degree “Managing Quality” in Specialized Master (ENSAM, Paris). As quality does not have a very good reputation in the startup world, it was necessary to transmit its strong points but also to adapt, innovate and advance step by step with the collaborators by training them, some of them having never had any experience in the medical field. The management team has always supported the quality process: it has appointed the pilots in a management review and set ambitious quality objectives. My role consisted first of all in establishing a relationship of trust with our service providers, our notified body BSI, as well as an ecosystem of internal and external skills to achieve our quality objectives.
Impact.
The quality process has had several positive impacts during its implementation. On the one hand, the “open” quality management system (QMS)[1] has facilitated the market access of melomind, a connected audio EEG device now marketed to learn how to manage one’s state of relaxation via the neurofeedback principle. On the other hand, it served as a springboard to develop our medical activity with the deployment of ISO 13485.
Methodology.
Today, we manage a totally dematerialized ISO 13485 QMS thanks to digital tools, which is a great advantage in the context of the health crisis: the first certification audits had to be conducted remotely. This specificity continues to be an advantage, since we can manage and improve our QMS remotely by maintaining a flexible organization in telecommuting.
Perspectives.
Our ISO 13485 process is conducted in parallel with the CE marking process according to the medical device regulation. Regulation (EU) 2017/745 of the European Parliament and of the Council of 5 April 2017 on Medical Devices (MDR) came into force on 25 May 2017 and came into effect on 26 May 2021 following the amending regulation (EU) 2020/561, i.e. 1 year later than initially planned. Our medical devices will be CE marked under the new MDR. BSI is one of the first organizations to be notified under the regulation. The marking of our medical devices is in progress with the collaboration of the whole team for the constitution of the technical file.
References.
[1] Duffaure S., Maranzana N., Attal Y. & Duchamp R. (2017). Démarche qualité et start-up. Déploiement au sein d’une start-up dans le domaine de la santé et du bien-être. Conférence : 12ème Congrès International de Génie Industriel (CIGI’17), Compiègne, France, Mai 2017.
Related Articles
Get in touch
We are glad you are interested in reaching out to us. Whether you have a question about our products or want to give us feedback, we are here to help.